An Efficient Genome-Wide Fusion Partner Screening System for Secretion of Recombinant Proteins in Yeast.
Rarely issued to produce recombinant proteins in the yeast Saccharomyces cerevisiae, we developed a new filtration system optimal genome-wide translational fusion partner (TFP), which involves the secretion signal optimal recruitment and fusion partner.
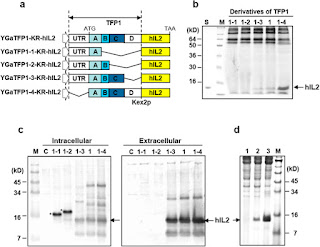
A TFP library constructed from genomic and Multi-species Recombinant Proteins cDNA libraries were cut using a technique based invertase signal sequence trap. The efficiency of the system was demonstrated using two rarely secreted protein, human interleukin (HIL) -2 and Hil-32. TFPs optimal for the secretion-2 and Hil Hil-32 very easily, resulting in secretion of this protein to hundreds mg / L.
In addition, many expressed yeast secretion signal and fusion partners are identified, leading to the efficient secretion of recombinant proteins. TFPs been found to be useful for a hypersecretion of other recombinant proteins in yields of up to several g / L. This screening technique could provide a new method for the production of various types of hard-to-express proteins.
the synthesis of recombinant proteins in microbial subunit vaccines: the role of yeast?
OBJECTIVE
recombinant protein subunit vaccines are formulated using protein antigens that have been synthesized in heterologous host cells. Some host cells are available for this purpose, ranging from Escherichia coli to mammalian cell lines. This article highlights the benefits of using yeast as the host recombinant.
RESULTS
Yeast species, Saccharomyces cerevisiae and https://gentaur.be/ Pichia pastoris, have been used to optimize yield potential for the development of functional antigen subunit vaccines against various diseases caused by bacteria and viruses. Saccharomyces cerevisiae has also been used in the manufacture of 11 approved vaccine against hepatitis B virus and one against human papillomavirus; in both cases, highly immunogenic recombinant protein to form virus-like particles.
CONCLUSION
Advances in our understanding of how cells respond to load yeast metabolism producing recombinant proteins will allow us to identify the strain of hosts that have improved properties and allows the synthesis of the results of the more challenging antigens that can not be produced in other systems. Therefore, yeast has the potential to become an important host organisms for the production of recombinant antigens can be used in the manufacture of subunit vaccines or in the development of new vaccines.
Comments
Post a Comment